Determine the Molarity of a Solution
Molarity moles of solute liters of solution. The resultant is the vector sum of these three vectors.

How To Calculate Molarity Using Solute Mass Chemistry Study Com
Grams active solute per.

. We can rearrange this equation to get the number of moles. To find pH for a given molarity you need to know how to work with logarithmic equations and a pH formula. 1 Calculate moles of NaF.
Determine the magnitude and direction of Maxs overall displacement. Find the percentage of the ripened apples if the number of ripe apples is 10 and the total number of apples in the basket are 60. This molarity calculator is a tool for converting the mass concentration of any solution to molar concentration or recalculating grams per ml to moles.
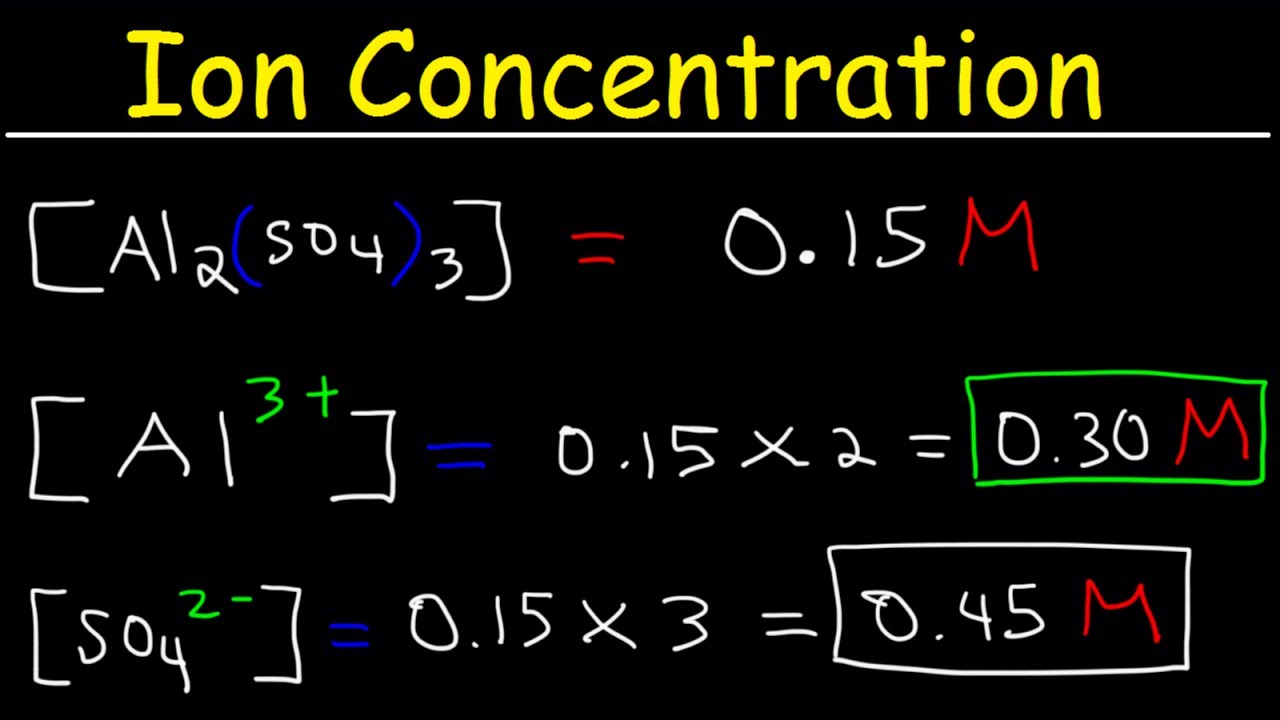
In chemistry a solutions concentration is how much of a dissolvable substance known as a solute is mixed with another substance called the solvent. Note that we often use square brackets around the formula of solvated species to indicate the molarity of a solution the concentration of the solution in mol L-1. N M V Example.
The official symbol for molarity is c concentration but many people use the old symbol M. The lab allows students to select from hundreds of standard reagents aqueous and manipulate them in a manner resembling a real lab. It is measured by considering two indicators that is the number of moles that are present in the.
Arrows very near to each other possibly nowhere near the bullseye indicate a high degree of precision. Molarity expresses the relationship between the number of moles of a solute per liters of solution or the volume of that solution. We can determine when the titration is complete by employing an acid-base indicator which will change color in a basic solution.
1 gram of NaCl is dissolved into 005 L of water so the normality of the solution is. To understand the topic as a whole you will. To compute any of the elements in the molarity equation you need to input the other three and choose the desired measurement unit.
Number of ripe apples 10. For example an aqueous solution of sodium chloride NaCl aq with a molarity of 0154 mol L -1 or 0154 mol dm -3 could be represented as. You can also calculate the mass of a substance needed to achieve a desired molarity.
Accuracy and Precision. 3 Calculate molarity of diluted solution. Determine water content without addition of dried methanol and if required adjust the water content to between 01 and 02 by adding either acetic anhydride or water allow to stand for 24 hours.
Correct The solution is 50 Molar. Calculate the molarity of a solution prepared by dissolving 237 grams of KMnO 4 into enough water to make 750 mL of solution. What is the molarity of a solution made by dissolving 34 g of KMnO 4 in 52 liters of water.
Given the total number of apples 60. 300 mol in 1000 mL so 3 x 1801000 054 mol in 180 mL. PH pOH 14.
Molarity also known as the molar concentration of a solution is the technique of calculating the amount of substance a particular chemical solution contains. In formula form molarity is expressed as. Volume expressed in either liters milliliters or microliters.
If the ratio is vv that means such quantity of solvent will be mix with another quantity of solvent then qs. This is derived from the molarity of protons hydrogen ions or H in the solution. Accuracy and precision are two separate concepts.
An increase in the molarity of a solution causes a considerable decrease in its pH that could be noticed by using a pH calculator from molarity. The Virtual Lab is an online simulation of a chemistry lab. You can also determine the pH from pOH as below.
Sodium chloride has a valence of 1 and a molecular weight of 58443. Eg 5 vv ethanol in water. To assist in the discussion the three vectors have been labeled as vectors A B and C.
Please scroll below to find our collection of. The pH scale ranges from 0 to 14 under usual conditions and measures the acidity of an aqueous solution. In chemistry the most commonly used unit for molarity is the number of moles per liter having the unit symbol molL or moldm 3 in SI unit.
1 Convert ppm to a gram-based concentration. It is designed to help students link chemical computations with authentic laboratory chemistry. PH 14 pOH.
Molar concentration also called molarity amount concentration or substance concentration is a measure of the concentration of a chemical species in particular of a solute in a solution in terms of amount of substance per unit volume of solution. This article will provide you with the molarity definition and the molarity formula. Molarity is the number of moles of a substance in one litre of solution.
025 10 25. Normality is often used in acid-base reactions or when dealing with acids or bases. Molarity is used mainly in chemistry when you know the chemical makeup of the solute youre using.
Therefore 25 of 10 is 25. This is a great chemistry tool for those who need to determine a solutions molar concentration from the mass volume and molecular weight. Therefore the equivalent weight is 584431 or 58443.
Calculate the molarity of a dye concentration given the molar mass is of the dye 327 gmol and a dye concentration of 2 ppm. M nV where n is the number of moles and V is the volume in litres. The product of molarity and volume of the sodium hydroxide provides the moles of the solution and the moles are equal in the acetic acid when completely.
More information and offline downloads. Last part would be. What is the pOH of a solution at 25texto.
Molarity M-is the molar concentration of a solution measured in moles of solute per liter of solution. Remember that of means times So 25 of 10 25100 10. Titrate 20 ml of hydrochloric acid Volumetric Solution of the same molarity as the solution being examined with the sodium hydroxide solution.
At the equivalence point the number of moles acid will equal the number of moles baseThus we can determine the molarity of the acid by. A head-to-tail vector addition diagram reveals. 064 M and 38 lower than claim First you want to start by using the titration information to find the molarity of the acetic acid.
Normality is similar to molarity except it expresses the number of active grams of a solute per liter of solution. The classic illustration distinguishing the two is to consider a target or bullseye. Dissolve sodium chloride NaCl in water.
Arrows surrounding a bullseye indicate a high degree of accuracy. The molarity definition is based on the volume of the solution NOT the volume of water. How to Calculate Normality of a Chemical Solution.
Incorrect The solution is 50 Molarity. 2 Calculate moles in 180 mL of resulting solution. It is prepared using a standard substance such as a primary standardStandard solutions are used to determine the concentrations of other substances such as solutions in.
Divide the mass of the solute. This example has neither the moles nor liters needed to find molarity so you must find the number of moles of the solute first. Level 1- Given moles and liters.
As soon as very faint pink color persist you have reached the equivalence point. A known mass of solute is dissolved to make a specific volume. M_1V_1M_2V_2 Where 1 is the acetic acid and 2 is the sodium hydroxide.
2a Using 0002 gL caculate the molarity. If I took 180 mL of that solution and diluted it to 500 mL determine the molarity of the resulting solution. In analytical chemistry a standard solution is a solution containing a precisely known concentration of an element or a substance.
1256 g 419 gmol 300 mol. As is the usual case the solution begins with a diagram of the vectors being added. 2 ppm 2 mg dye L of solution.
This is the gram equivalent weight of solute per liter of solution. Determine the total mass of the solution in grams.

Molarity Calculator Graphpad Deals 53 Off Www Ingeniovirtual Com

Question Video Calculating The Molarity Of A Solution From Mass And Volume Nagwa

Comments
Post a Comment